New Mayo Clinic study finds mild to severe atrophy in testes of boys on puberty blockers
The authors of the preprint study express doubt in 'reversibility' claims of puberty blockers for gender dysphoric children.

In a groundbreaking study from the Mayo Clinic, a globally recognized leader in medical research and patient care, researchers examined the effects of puberty blockers on testicular development in gender dysphoric male children. Their investigation revealed evidence of mild to severe atrophy in the sex glands of these children, leading the authors to express doubt in the claims of “reversibility” often made about puberty blockers.
The authors assert, “We provide unprecedented histological evidence revealing detrimental pediatric testicular sex gland responses to [puberty blockers].”
This preprint study, not yet peer-reviewed, presents evidence that puberty blockers induce significant cellular changes, impacting testicular development and sperm production in ways that are not fully reversible, with potentially permanent effects on testicular function and fertility. It challenges the longstanding view of puberty blockers as a reversible "pause button" on puberty.
As noted by the researchers of this study, no long-term studies exist for the use of puberty blockers in the context of stopping puberty for gender dysphoric children, and many potential health consequences remain unknown. In particular, the long-term impact on reproductive health is uncertain, making this study critical for filling this knowledge gap.
To address these unknowns, the Mayo Clinic has established the largest collection of testicular samples for patients aged 0-17 years, including those with gender dysphoria who have and have not yet received puberty blocker treatment, creating a database of over 130,000 individual cells for analysis.
Using a novel approach, the research team meticulously analyzed testicular tissue samples from youths undergoing puberty blocker treatment, with those not on puberty blocker treatment serving as controls. This comparison provides important insights into the potential cellular and molecular changes induced by these drugs.
Key Findings
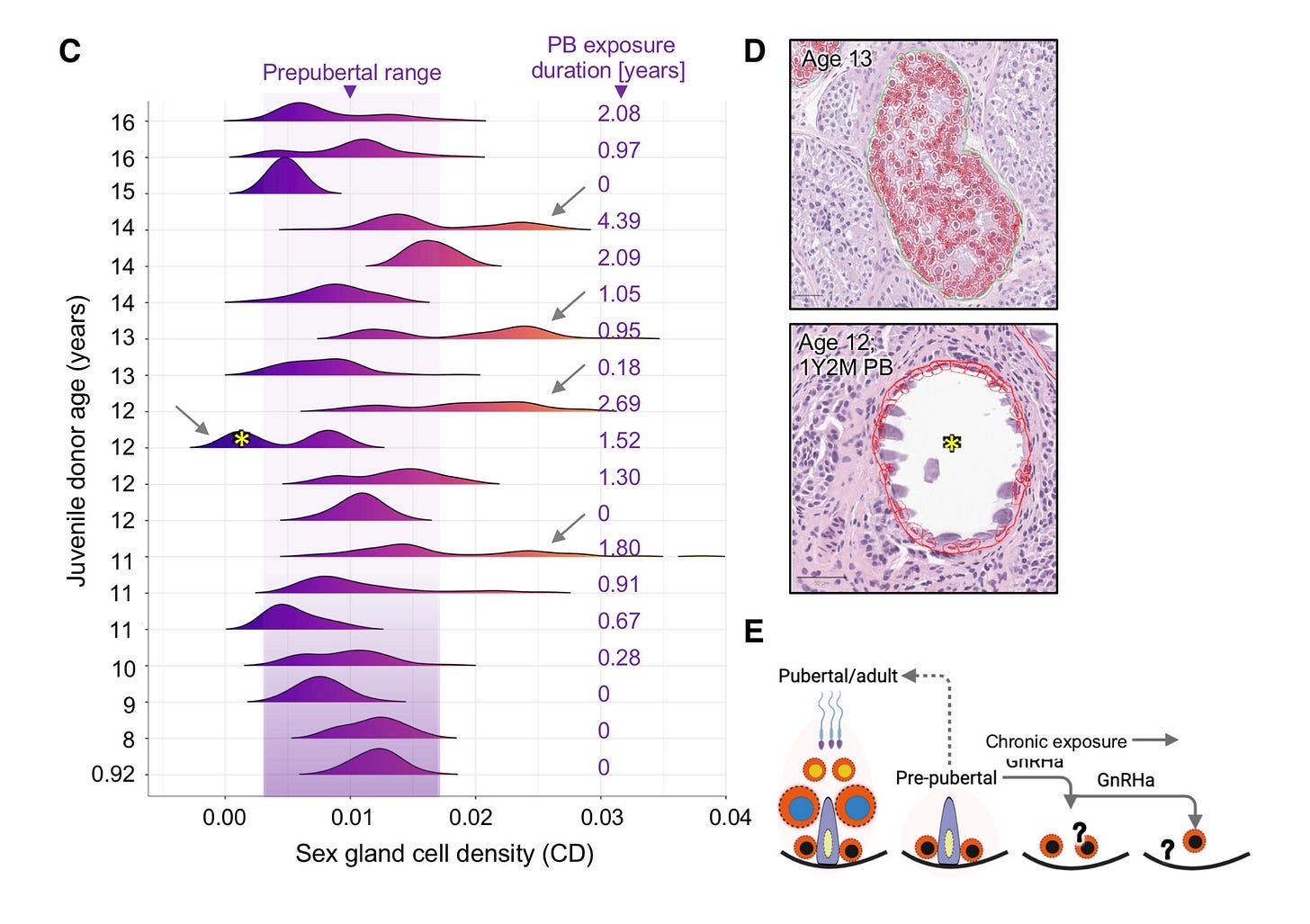
The study utilized the Mayo Clinic's Pediatric Testicular Biobank for Fertility Preservation, which has been recruiting patients primarily from pediatric urology departments since 2015. Researchers analyzed testicular specimens from 87 young individuals (ages 0-17) undergoing fertility preservation surgery for various health reasons. Among these, 16 were gender dysphoric boys between the ages of 10 and 16, all of whom began identifying as transgender girls between the ages of 2 and 15. At the time of surgery, 9 patients (56%) were already on puberty blockers, with exposure ranging from 3 to 52 months. The authors noted that 100% of the 16 children would eventually go on to take them, highlighting “the widespread nature of PB intervention in this demographic.”
Among nine patients treated with puberty blockers, two exhibited unusual features in their testicles upon physical examination. One patient had abnormalities in both testicles, including incomplete development of the tunica albuginea, which is a protective covering around the testicles. The other patient had a right testicle that was difficult to detect.
In one part of the tissue-level analysis, over 400 testicular biopsy samples were analyzed and stained to examine the differences between those treated with puberty blockers and those who were not. Comparisons showed that testicular development in those treated with puberty blockers was abnormal compared to non-treated individuals. There was variability in how individuals responded to puberty blockers, leading to different outcomes in testicular development, including the degeneration of testicular tissues.
The study authors presented a case of a 12-year-old patient who underwent treatment with puberty blockers for 14 months. In this individual, 59% of the sex glands showed complete atrophy, along with the presence of microlithiasis—a condition where small clusters of calcium form in the testicles. This insight suggests that puberty blockers could lead to lasting structural changes. Additionally, research has shown a link between testicular microlithiasis and testicular cancer.
This study also utilized single-cell analysis to investigate the effects of puberty blockers and aging on testicular cell composition. It took a very detailed look at individual cells from the testicles of a 14-year-old who had been on puberty blockers for over 4 years. The study analyzed a total of 130,100 cells, including 11,199 cells from the juvenile puberty blocker-treated patient.
The study observed that over 90% of the cells responsible for sperm production in this patient were stunted at an early developmental stage, unable to progress further. Additionally, it found "pathologically" higher and lower levels of two types of support cells (Sertoli cells) necessary for healthy sperm development. These findings suggest that puberty blockers can disrupt the normal maturation process of cells critical for sperm production.
In another part of the analysis, the authors found distinct cell-specific changes, including altered expression patterns of puberty-associated genes in endothelial cells, due to puberty blocker treatment. The authors believe that these drugs might induce juvenile testicular atrophy in part by disrupting the normal function of testicular endothelial cells.
Another aspect of the study focused on examining the effects of puberty blockers on the genetic activity of early-stage sperm cells, revealing significant changes that could potentially influence their development and fertility. By analyzing the activity of specific genes within these cells, the researchers found that puberty blockers may have caused alterations in gene expression, affecting processes crucial for the normal growth and function of these cells. This analysis suggests that the use of puberty blockers in gender dysphoric youth could have lasting implications for their reproductive health, particularly by impacting the ability of these early-stage sperm cells to mature properly.
Study Impact
Puberty blockers are increasingly used as a treatment for gender dysphoric youth to halt the development of secondary sex characteristics, such as breast development and widening of hips in females, or the growth of facial hair and deepening of the voice in males. Thousands of children in the United States are placed on this medical pathway as part of the gender-affirming model of care, under the presumption that these drugs are safe and fully reversible.
However, many aspects of the long-term consequences of puberty blockers, which have been administered to children off-label in an experimental manner, remain unknown. This study contributes valuable insights into the potential irreversible harm these treatments can cause to bodily and reproductive functions.
Arguably, the most critical finding is the evidence of mild to severe sex gland atrophy in children treated with puberty blockers. This atrophy signifies potential damage or impairment to the structures essential for sperm production, raising serious concerns about the long-term fertility impacts of these drugs for these individuals.
Given the Mayo Clinic's esteemed reputation in the medical and research communities, should the study pass peer review without any issues, its findings will carry significant weight.
Broader Implications
Puberty blockers belong to a group of synthetic gonadotropin-releasing hormone
(GnRH) analogues. These drugs act on the pituitary gland to hinder the release of chemical signals that typically trigger the production of estrogen and testosterone. Historically GnRH analogues were used to treat conditions such as prostate cancer, fibroids, and endometriosis and, in some cases, as a measure to chemically castrate sex offenders.
In children, puberty blockers prevent the natural changes of puberty driven by sex hormones and have been used to treat central precocious puberty, a condition where a child begins to sexually mature much earlier than usual. In gender dysphoria, puberty blockers are administered experimentally, lacking long-term testing.
Notably, the U.S. Food and Drug Administration (FDA) has not approved puberty blockers and sex hormones for use in pediatric gender care. No clinical trials have substantiated the safety of these drugs for such non-approved applications and manufacturers of puberty blockers have repeatedly declined to conduct safety trials for their use on this cohort.
While puberty-blocking drugs are often promoted as “safe,” "reversible" and a "pause button" on puberty, these characterizations seem to stem from their approved use for treating central precocious puberty in younger children, not their burgeoning off-label use for managing gender dysphoria in adolescents.
Past studies have indicated possible negative effects on bone density and brain health. There is also a concern that these drugs might solidify gender dysphoria in adolescents, potentially leading them down a lifelong road of biomedical interventions. Following reports in 2016 of suicidal ideation in children administered puberty blockers, the FDA instructed drug manufacturers to include a warning about potential psychiatric issues on the drugs' labels.
Puberty blockers are increasingly administered to adolescents at Tanner Stage 2, the first signs of puberty. Research shows administering puberty blockers at this stage, followed by cross-sex hormones, may result in infertility, sterility, and sexual dysfunction. Furthermore, they inhibit the development of mature male genitalia, making it difficult to create a pseudovagina in the event of a later vaginoplasty due to a lack of sufficient tissue.
The National Health Service England recently announced it would no longer prescribe puberty blockers to youth outside of research settings and closed down its only national clinical service for pediatric gender medicine, following a review that deemed the service "not safe.”
Several European countries, including Sweden, Finland, the UK, Denmark, and Norway have updated their guidelines for youth transition to align with systematic evidence reviews, the gold standard in evidence-based medicine. These reviews concluded that the risks associated with youth transition outweigh any purported benefits. Consequently, these countries have implemented restrictions on medical interventions, prioritizing psychotherapy as a first-line response for minors experiencing gender-related distress.



But they said! No harm! No problem!
The no harm, reversible messaging came from medical educators. Do not know if they have changed their tunes of late, but perhaps this should be considered.
so not reversible?